Innovative anaphylaxis treatment now approved for UK
Following MHRA approval in July 2025, EURneffy is the UK's first needle-free alternative to adrenaline auto-injectors. Our specialist team provides comprehensive anaphylaxis management and will be ready to prescribe this innovative nasal spray treatment when it becomes available.





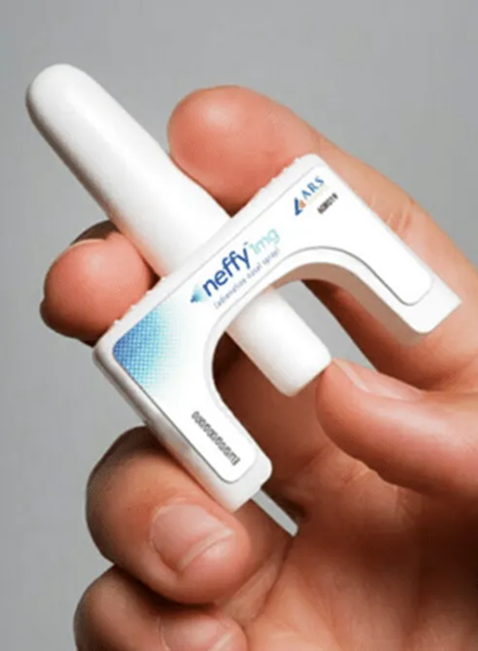





Clinical studies show this approach is particularly beneficial for children, needle-phobic patients, and emergency responders from the public who may hesitate with traditional injection devices.
Evaluate your current anaphylaxis risk and emergency action plan effectiveness
Conduct individual assessment to determine whether EURneffy is suitable alongside or instead of traditional auto-injectors
Provide comprehensive training on proper EURneffy administration techniques when prescribed
Develop updated emergency plans incorporating the most appropriate adrenaline delivery system for your needs

EURneffy is a newly MHRA-approved, needle-free adrenaline (epinephrine) nasal spray for the emergency treatment of severe allergic reactions, including anaphylaxis.
It is expected to be available in late 2025 as an alternative to auto-injectors like EpiPen or Jext for suitable older children, teenagers and adults.
The spray delivers a 2mg dose of adrenaline through the nasal lining, where it’s rapidly absorbed into the bloodstream. Once active, it works to raise blood pressure, open the airways, and quickly calm the immune system’s response.
A lower-dose 1mg version, intended for younger children as an alternative to junior auto-injectors, is also in development and expected to follow.
As with all adrenaline devices, EURneffy won’t be suitable for everyone, and proper training and clinical guidance will be essential once available.


















